Chronic Obstructive Pulmonary Disease (COPD)
COPD is a highly prevalent, life-threatening lung disease that is among the leading causes of mortality and disability worldwide, yet underdiagnosis is frequent and many unmet needs remain in its management.1-3
PART 1
Understanding COPD
What is COPD?
Chronic obstructive pulmonary disease (COPD) is a common lung disease causing restricted airflow and increasingly severe breathing difficulties.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines COPD as a heterogeneous lung condition characterised by chronic respiratory symptoms due to abnormalities of the airways and/or alveoli that cause persistent, often progressive airflow obstruction.3
Key symptoms
Symptoms include dyspnoea, cough and sputum production.
Although COPD is a chronic disease, a substantial number of patients suffer from acute exacerbations of COPD (AECOPD), which are defined by GOLD as an event characterised by increased dyspnoea and/or cough and sputum that worsens in under 14 days which may be accompanied by tachypnea and/or tachycardia and is often associated with increased local and systemic inflammation caused by infection, pollution, or other insult to the airways.3
Data from various patient cohorts indicates that up to 70% of patients experience at least one AECOPD over a 1–5 year follow-up period.4
AECOPD often require urgent care, escalation of medication and/or hospitalisation, incurring substantial additional costs.5
Pathophysiology
COPD is thought to be caused by a mixture of genetic and environmental factors, namely tobacco smoking and exposure to pollution (occupational exposure and indoor pollution from burning wood and other biomass fuels).3
As COPD is a heterogeneous disease, an expert panel of specialists recommends classifying COPD based on underlying causative mechanisms, in order to develop personalised treatment approaches.2
Disease burden
COPD creates a substantial economic1 and social burden.6, 7
COPD impacts patient quality-of-life (QoL) & mental well-being: COPD is associated with poorer physical health and disease-specific health status (vs. individuals without COPD),8 and a high incidence of psychiatric comorbidities9 including depression.10, 11
COPD is the 3rd leading cause of mortality worldwide and 7th leading cause of morbidity.12
COPD patients are at risk of frequent hospitalisations, which are associated with emotional distress: nearly 1 in 5 patients attending the emergency department are hospitalised at least once in the year following their initial visit.13
AECOPD are associated with lung function decline14, worsening QoL,15, 16 decreased physical activity,17 depression,18 re-hospitalisations, further AECOPD and mortality.19, 20
Comorbidities
There is a high burden of comorbidities, particularly cardiovascular and diabetes3:
- The most frequent are hypertension, coronary artery disease, diabetes, osteoarthritis, psychiatric disease, asthma and obstructive sleep apnoea (OSA)21, 22
- Comorbidities can influence a patient’s clinical condition and prognosis and require treatment: some conditions such as heart failure, ischaemic heart disease, sleep disorders and depression/anxiety are associated with AECOPD.3, 23 OSA may worsen night-time hypoxemia and increase the risk for pulmonary hypertension22
Physical deconditioning
COPD patients often have a ‘downward spiral’ of health: systemic effects lead to cardiovascular comorbidities, muscle wasting and osteoporosis, leading to further inactivity and physical deconditioning. Similarly, exacerbations lead to decreased lung function, physical inactivity and mental health decline.24

Understanding COPD: summary
COPD is a progressive lung disease, which manifests primarily with airflow obstruction and is associated with reduced patient QoL and increased mortality risk. So how common is COPD and how is it diagnosed?
PART 2
Prevalence and diagnosis
Prevalence
Prevalence estimates vary, however ~480 million people are thought to be living with COPD worldwide25 and the incidence increased by >85% between 1990 and 2019,26 with further increases projected in the coming decades due to the continued exposure to risk factors, an aging population and improved awareness and diagnosis.3
Diagnosis
Signs and symptoms of COPD can vary between patients and on a daily basis. However a diagnosis should be considered in any patient with persistent dyspnoea and chronic cough (intermittent or continuous) with or without sputum production and a history of recurrent lower respiratory tract infections and/or exposure to risk factors such as smoking or pollution.3
The gold standard diagnostic method is spirometry testing (presence of airway limitation that is not fully reversible with a post-bronchodilator FEV1/FVC ratio of <0.7) together with clinical history and presence of risk factors.3
Supporting assessments to exclude differential diagnoses may include imaging (chest X-ray or CT scan), arterial blood gases (to detect hypoxia / hypercapnia) and sputum examination (useful in patients presenting with an AECOPD).3
Diagnosis: unmet needs
Globally, there is a large variation in the reported prevalence of COPD, with 10–95% of patients underdiagnosed, often due to under, or inconsistent use of, diagnostic methods or lack of availability,27 with some patients unable to access diagnostic facilities or appropriate healthcare.
Several weaknesses in the current diagnostic methods and criteria have been highlighted by experts. Post-bronchodilator spirometry is not predictive of symptoms and does not detect early pathological changes. Moreover, spirometry is often underused or misinterpreted. An expert panel suggests that the use of modern techniques such as imaging could be utilised to detect mild disease before irreversible pathological changes have occurred.2
Severity grading
To guide treatment approach, GOLD recommend grading COPD by level of airflow obstruction (GOLD 1–4) and by symptoms/exacerbation history3:
- The level of airflow obstruction ranges from mild (GOLD 1) to very severe (GOLD 4) depending on the predicted FEV1
- Symptom severity ranges from GOLD A (low symptom burden: mMRC 0–1 or CAT<10, low exacerbation history: ≤1 moderate exacerbation) and GOLD B (high symptom burden: mMRC ≥2 or CAT ≥10, low exacerbation history: ≤1 moderate exacerbation), to GOLD E (any symptom burden, high exacerbation risk: ≥2 moderate exacerbations or ≥1 leading to hospitalisation)

Prevalence and diagnosis: summary
COPD is a heterogeneous, highly prevalent lung condition. Whilst symptoms may be variable, the gold standard for diagnosis is the post-bronchodilator FEV1/FVC ratio. Exacerbation history and symptom burden are also assessed to determine disease severity. So how are severity gradings used to inform treatment choice?
PART 3
Treatment and prognosis
Treatment goals
Choice of COPD treatment is dependent on disease severity* and aims to alleviate symptoms whilst reducing disease progression, exacerbations and mortality.
Interventions include lifestyle changes, pharmacologic therapy and oxygen/ventilatory support, such as long-term oxygen therapy and high-flow therapy (Figure).
*The 2023 GOLD COPD report recognises three symptom severity groups – “A”, “B”, and “E”.3
Guidelines
The information in the following sections is not intended to replace local guidelines or recommendations from expert societies. Please check the latest local/international guidelines to inform treatment decisions. Some relevant links are listed below:
https://goldcopd.org/2023-gold-report-2/
Behavioural / lifestyle modification
Behavioural and lifestyle changes include smoking cessation and pulmonary rehabilitation (exercise training combined with patient education).
Both of these strategies have been shown to reduce mortality in patients with COPD.3
Pharmacologic treatments
Pharmacologic treatments can reduce symptoms and AECOPD frequency and severity, and improve health status and exercise tolerance.3
Regimens should be individualised based on symptom severity, exacerbation risk, side-effects, patient comorbidities and drug availability/cost, as well as patient preference.3
GOLD recommend inhaled bronchodilators as central to symptom management, these drugs work to relax or prevent the contraction of smooth airway muscle.3
Antibiotics may be prescribed for patients during, or to prevent, AECOPD,28, 29 Patients should also receive immunisations to prevent infections that could lead to AECOPD.3
Mucolytics reduce AECOPD risk in some patients and are recommended by ERS/ATS for patients with moderate or severe airflow limitation and AECOPD despite optimal inhaler therapy.3, 29
Oxygen therapy
O2 therapy can provide relief from hypoxemia and reduce the work of breathing. Long-term O2 therapy should be reassessed regularly and is recommended by GOLD and ATS for patients with severe resting hypoxemia, in whom it is associated with a small increase in survival.3, 30, 31
O2 may be delivered through an oxygen concentrator, compressed or liquid oxygen cylinder or via a ventilator.
High-flow therapy (HFT)
HFT delivers a humidified, warmed mixture of air, with or without O2 and may benefit patients with chronic cough and mucus production, symptoms that can be difficult to manage with standard therapies alone.32
HFT is designed to be administered via a nasal high-flow cannula (NHFC). Home HFT can supply a flow of 10–60L/min or 10–15L/min if O2 is added. The configuration used depends on the needs of the patient.
Some of the benefits of HFT include improved secretion management,33 reduced dyspnoea,34-36 reduced dead space,37 improved patient comfort33, 37 and reduced AECOPD.32, 38
Read more about HFT here and the benefits of HFT for patients with COPD here.
Home non-invasive ventilation (NIV)
NIV is recommended by GOLD and ERS for stable patients with severe chronic hypercapnia and a history of hospitalisation for acute respiratory failure.3, 39-41
- Benefits include improved survival and QoL and reduced risk of hospital admission39, 40
- Following an AECOPD, NIV combined with home O2 therapy has been shown to significantly prolong the time to readmission or death and reduce further AECOPDs40 and is cost-effective42
- Clinical trial data highlight the importance of carefully evaluating patient phenotype and the timing and delivery of home NIV to ensure the selection of patients most likely to benefit43
Flow diagram showing patient selection for home NIV based on clinical trial data.39, 40
Read more about the clinical evidence and patient selection for home NIV here.
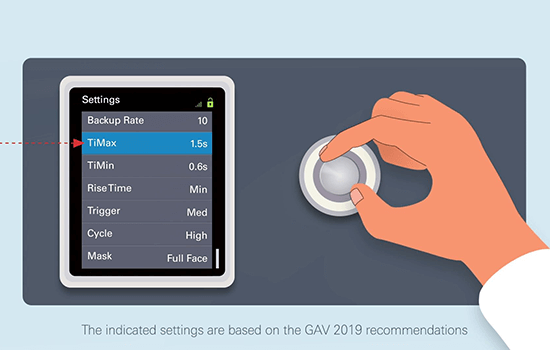
Home NIV best practices
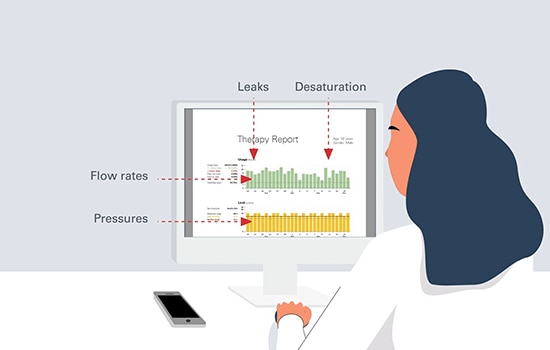
Considerations for home NIV best practice include careful selection of patients most likely to benefit from treatment, delivering therapy that is effective and well-tolerated and careful monitoring of treatment effects.
Improving elevated arterial carbon dioxide levels should be one of the main goals of home NIV and clinical trial data show that higher inspiratory pressures contribute to the success of home NIV therapy.39, 40, 44
NIV for acute events
GOLD also recommend NIV for the treatment of AECOPD where there is respiratory acidosis / severe dyspnoea with clinical signs suggesting respiratory muscle fatigue / persistent hypoxemia despite supplemental O2.3
ERS/ATS recommend NIV for acute-on-chronic hypercapnic respiratory failure due to AECOPD.28
Cost effectiveness of NIV initiation at home vs in-hospital
Choice of LTOT, HFT or NIV
LTOT, home HFT and home NIV can be used separately or in combination for the home-based treatment of COPD patients. Some of the benefits of each approach are shown in the table.
Continuous positive airway pressure (CPAP)
CPAP may also benefit some patients and has been shown to improve survival and reduce the risk of hospitalisation in patients with both COPD and obstructive sleep apnoea.3
Surgery
Surgical options include lung volume reduction, bullectomy and lung transplantation.
Prognosis
Whilst COPD is a progressive disease, appropriate treatment can reduce symptoms, exacerbation frequency and severity, and improve health status and exercise tolerance.3
Some treatments may also slow the rate of lung function decline and reduce mortality.3
Long-term O2 therapy has been shown to improve survival in patients with severe resting hypoxemia and NIV decreases mortality and prevents re-hospitalisation in patients with severe chronic hypercapnia.3
Untreated COPD may lead to acute or chronic respiratory failure. Patients are particularly at risk during an AECOPD since an increase in airway obstruction leaves the respiratory system vulnerable to overloading.
Future outlook
There is an urgent need to do more to tackle COPD globally.46
An expert panel of specialists writing for the Lancet note that a coordinated international response (as was the case for COVID-19) can overcome existing barriers to treatment and yield rapid results. In particular, they advocate2:
- Public health strategies to ban smoking and maintain clean air
- A personalised medicine approach – based on a comprehensive assessment of disease pathophysiology and symptoms, as well as patients’ needs, capabilities and preferences
- Further investment in curative and regenerative therapies to move beyond largely symptomatic treatment options
- More focus on AECOPD which play a crucial role in disease progression and costs but tend to be imprecisely defined and insufficiently investigated
Read the infographics to further explore some of the key unmet needs in COPD care:

Treatment and prognosis: summary
COPD is a progressive disease which substantially impacts patient QoL and is a leading cause of morbidity and mortality worldwide.3, 6, 12 However appropriate treatment has been shown to improve symptoms, AECOPD frequency and severity,3, 32 and, in patients with COPD and chronic hypercapnia treated with non-invasive ventilation, survival.39
This content is intended for health professionals only.
**These ‘how to’ videos on the use of home NIV are for healthcare professionals only and are based on the 2019 GAV recommendations, which are themselves based on the literature and input from experienced respiratory therapists. The care protocol remains the responsibility of the prescribing physician and the device settings must be chosen with regard to each patient’s needs.
References
- Iheanacho, I., et al., Economic Burden of Chronic Obstructive Pulmonary Disease (COPD): A Systematic Literature Review. Int J Chron Obstruct Pulmon Dis, 2020. 15: p. 439-460.
- Stolz, D., et al., Towards the elimination of chronic obstructive pulmonary disease: a Lancet Commission. Lancet, 2022. 400(10356): p. 921-972.
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Prevention, Diagnosis and Management of COPD: 2023 Report 2023. Available at https://goldcopd.org/2023-gold-report-2/
- Hoogendoorn, M., et al., Prediction models for exacerbations in different COPD patient populations: comparing results of five large data sources. Int J Chron Obstruct Pulmon Dis, 2017. 12: p. 3183-3194.
- Dalal, A.A., et al., Impact of COPD Exacerbation Frequency on Costs for a Managed Care Population. J Manag Care Spec Pharm, 2015. 21(7): p. 575-83.
- Svedsater, H., et al., Life Impact and Treatment Preferences of Individuals with Asthma and Chronic Obstructive Pulmonary Disease: Results from Qualitative Interviews and Focus Groups. Adv Ther, 2017. 34(6): p. 1466-1481.
- Cook, N.S., et al., Patients’ perspectives on COPD: findings from a social media listening study. ERJ Open Res, 2019. 5(1).
- Franssen, F.M.E., et al., The physical, mental, and social impact of COPD in a population-based sample: results from the Longitudinal Aging Study Amsterdam. NPJ Prim Care Respir Med, 2018. 28(1): p. 30.
- FitzGerald, J.M., et al., Resource use study in COPD (RUSIC): a prospective study to quantify the effects of COPD exacerbations on health care resource use among COPD patients. Can Respir J, 2007. 14(3): p. 145-52.
- Omachi, T.A., et al., Depression and health-related quality of life in chronic obstructive pulmonary disease. Am J Med, 2009. 122(8): p. 778 e9-15.
- Zhang, M.W., et al., Prevalence of depressive symptoms in patients with chronic obstructive pulmonary disease: a systematic review, meta-analysis and meta-regression. Gen Hosp Psychiatry, 2011. 33(3): p. 217-23.
- GBD 2019 Diseases and Injuries Collaborators, Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet, 2020. 396(10258): p. 1204-1222.
- Yeatts, K.B., et al., Population-based burden of COPD-related visits in the ED: return ED visits, hospital admissions, and comorbidity risks. Chest, 2013. 144(3): p. 784-793.
- Donaldson, G.C., et al., Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax, 2002. 57(10): p. 847-52.