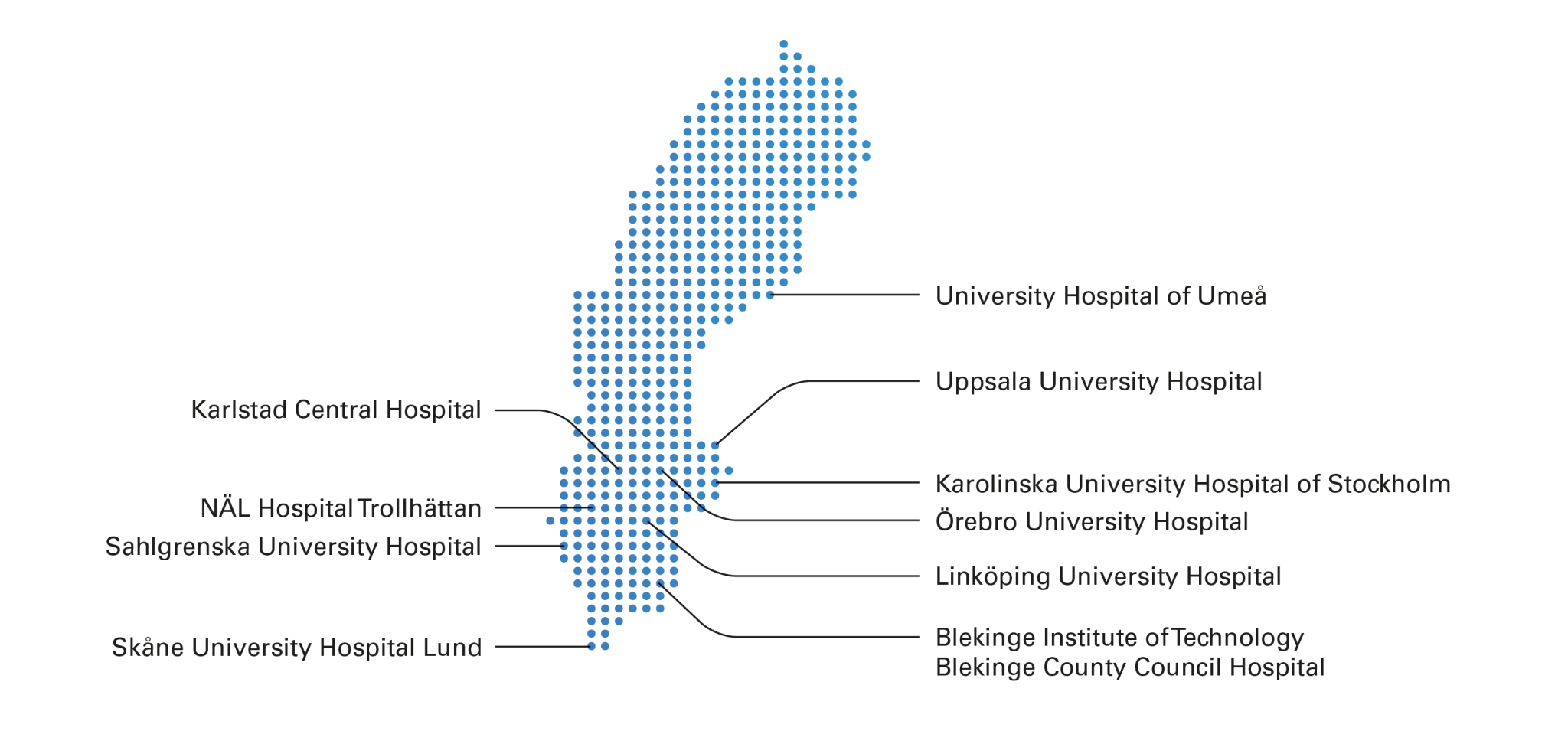
The HiLOT trial
Home high-flow therapy (HFT) has been shown to reduce acute exacerbation rates and hospital admissions in patients with chronic pulmonary obstructive disease (COPD)1,2.
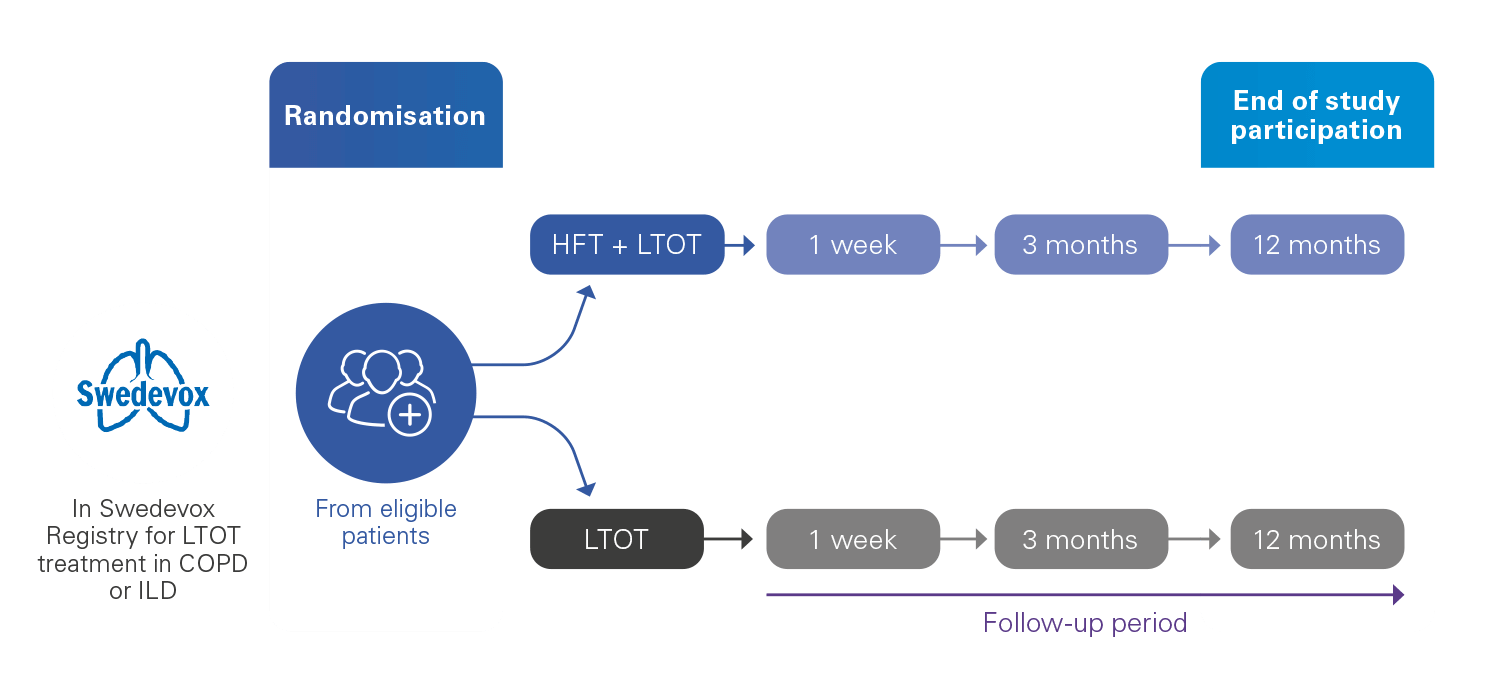
With the need to grow the level of evidence on the benefits of home HFT, HiLOT aims to evaluate the effect of using home HFT with long-term oxygen therapy (LTOT) during one year in people with COPD or interstitial lung disease (ILD), vs using LTOT alone.
Study objectives
The main objective is to evaluate the impact of home HFT with LTOT on mortality and hospitalisations in patients with COPD. The trial will also evaluate the benefit(s) of home HFT with LTOT on quality of life and symptoms and assess the cost effectiveness, compliance and tolerance of the therapy.
The study endpoints
Primary endpoint:
Time to first hospitalisation or all-cause death in the year following randomisation in COPD patients.
Secondary endpoints include:
- Symptoms
- Health-related quality of life
- Hospitalisations
- Exacerbations
- Cost effectiveness
Sign up for our clinical newsletter
Sign up to receive clinical updates about COPD, high-flow therapy, non-invasive ventilation, remote monitoring and more. You can also visit our Clinical News page for a selection of the latest research in these areas.
Support for investigator-initiated research
Resmed believes in the need to support ethical, independent clinical research, conducted by qualified third-party investigators.
This content is intended for health professionals only
*Please see https://www.clinicaltrials.gov/study/NCT06247397 for further information
**270 COPD patients and 40 ILD patients
References
- Storgaard LH, et al. Long-term effects of oxygen-enriched high-flow nasal cannula treatment in COPD patients with chronic hypoxemic respiratory failure. Int J Chron Obstruct Pulmon Dis 2018;13:1195-1205. DOI : 10.2147/COPD.S159666
- Rea H, et al. The clinical utility of long-term humidification therapy in chronic airway disease. Respir Med 2010;104:525-533. DOI : 10.1016/j.rmed.2009.12.016V
Content last updated: 04/2024